Pancreo-Lip®
¹³C mixed triglyceride breath test to determine pancreatic function
This ¹³C mixed triglyceride breath test is used to determine exocrine pancreatic insufficiency, which can lead to problems digesting and absorbing fat from food. Pancreo-Lip offers an accurate alternative to the stool test for lipid analysis. This test is also being clinically tested in preparation for approval.
The test is being made available for clinical research and INFAI welcomes inquiries from researchers and physicians interested in using the test.
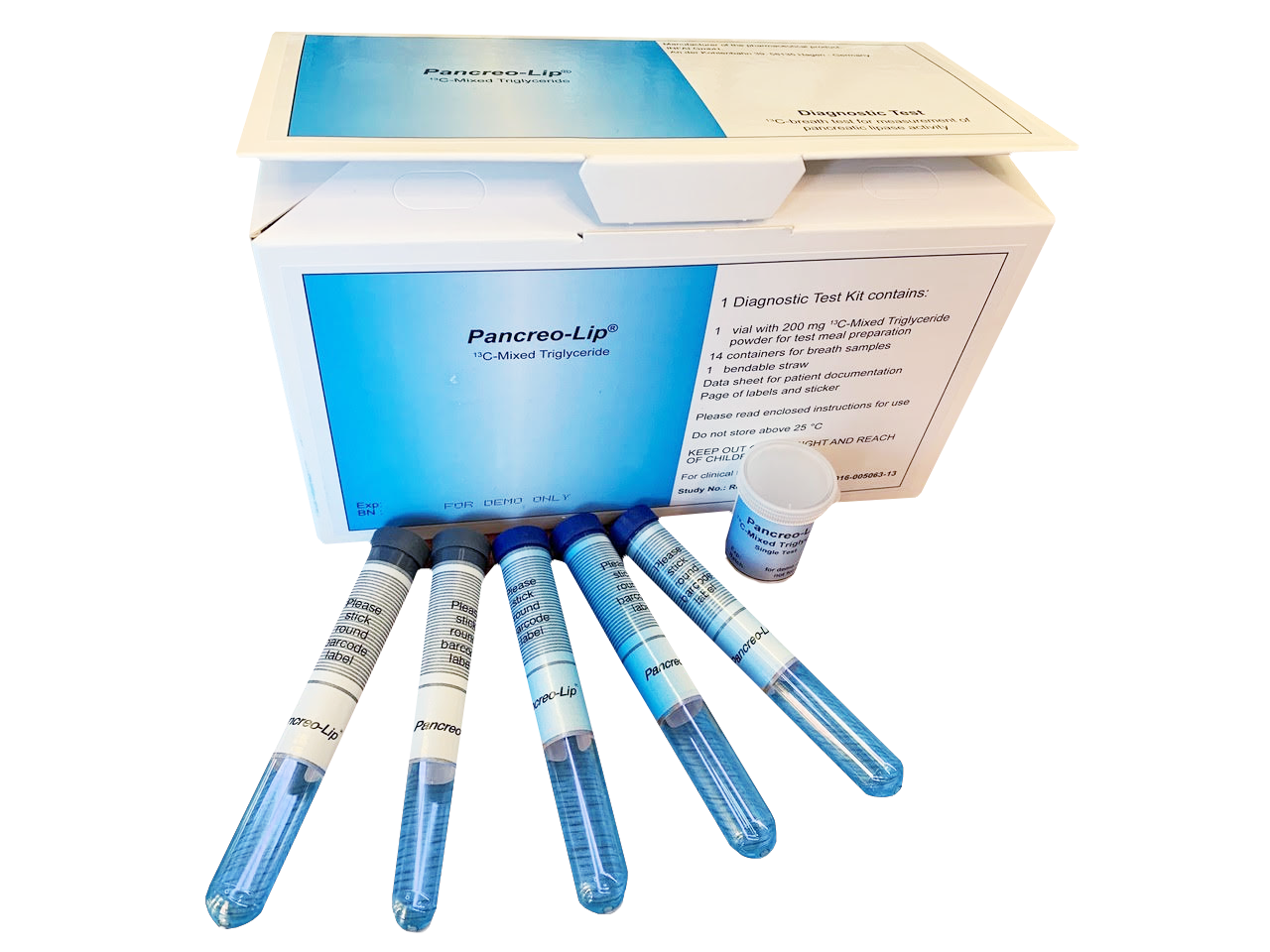
Features
Not radioactive
Cost-effective
Indications for use
A decrease in lipase activity is an early indicator of pancreatic dysfunction. Pancreo-Lip is a ¹³C mixed triglyceride breath test for the determination of pancreatic dysfunction by measuring lipase activity in vivo.
Direct pancreatic function tests such as the Secretin-Caerulein test are not suitable for routine diagnosis because they are time-consuming, expensive, invasive and not internationally standardized. Indirect tests such as the fluorescein dilaurate test, and fecal chymotrypsin activity have limited sensitivity. The determination of fecal elastase 1 is the most promising test currently in use, but all stool tests have their own disadvantages.
Vantrappen et al (Gastroenterology, Vol 96, 126-134, 1989) described for the first time a pancreatic lipase breath test using a ¹³C-labelled mixed triglyceride substrate. Since then a number of authors have further evaluated the test. Clinical studies in collaboration with INFAI (Löser et al. Scand. J. Gastroenterol. Vol 33, 327-334, 1998) showed that the test is a valuable diagnostic tool for the detection of pancreatic insufficiency.
INFAI is currently completing clinical studies for this test in preparation for the application for a pan-European marketing authorization. The kit is currently available on a per-patient basis and for research projects, subject to the appropriate ethical and licensing exclusions.
Test principle and protocol
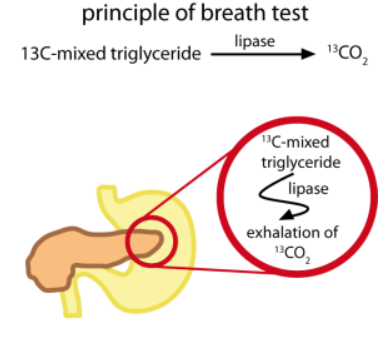
¹³C-Mixed Triglyceride (200 mg glyceryl-1,3-dioctadecanoate-2-octanoate-1-¹³C in pure powder form), mixed with chocolate cream and administered with toast and butter is hydrolyzed by pancreatic lipase. One of the products of this hydrolysis, ¹³C-octanoic acid, is rapidly broken down in the liver to ¹³CO₂ and released into the breath. The characteristics of the ¹³CO₂ excretion curve can be used to distinguish between normal and abnormal levels of pancreatic lipase activity.
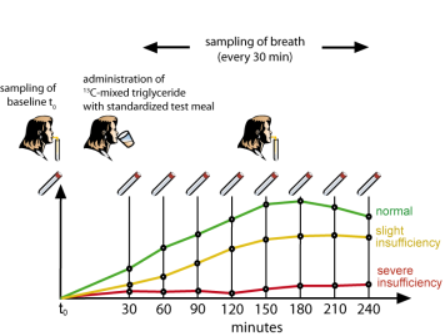
After six hours of fasting, the patient eats a standardized test meal consisting of ¹³C-mixed triglyceride (200 mg glyceryl-1,3-dioctadecanoate-2-octanoate-1-¹³C as a pure powder) mixed with chocolate cream (10 g) spread on a slice of toast (100 g) with butter (15 g). 150 ml of coffee, tea or water are given as a test meal.
The test protocol for Pancreo-Lip is convenient and patient-friendly and takes about 250 minutes. It is recommended that the patient sits during the test. The test begins with the sampling of the basal value. The patient breathes easily through a straw into a glass tube which is closed with a rubber stopper. Two basal values are recorded before the test meal is eaten. After consumption, another eight breath samples are taken at half-hour intervals. The sample tubes are returned to their original packaging and sent to a qualified laboratory for determination of ¹³CO₂ concentration.
The ¹³CO₂ excretion curve is analysed mathematically using a program developed by INFAI. This allows the identification of different levels of pancreatic insufficiency.
More Information
Publications
- Comparative clinical evaluation of the ¹³C-Mixed-Triglyceride breath test as an indirect pancreatic function test
Löser C., Brauer C., Aygen S., Hennemann O., Fölsch U.R; Scand. J. Gastroenterol. 33 (1998) 327-334
Patent
- EP1560603, Method for measuring pancreatic metabolism, European Patent, 07.03.2007
- US7762957, Method for measuring pancreatic metabolism, US Patent, 27.07.2010
- WO2004043498, Method for measuring pancreatic metabolism, International Patent, 27.05.2004
References
- Keller, Jutta et al. “European guideline on indications, performance and clinical impact of ¹³C-breath tests in adult and pediatric patients: An EAGEN, ESNM, and ESPGHAN consensus, supported by EPC.” United European gastroenterology journal vol. 9,5 (2021): 598-625.
- Capurso G, Traini M, Piciucchi M, Signoretti M, Arcidiacono PG. Exocrine pancreatic insufficiency: prevalence, diagnosis, and management. Clin Exp Gastroenterol. 2019; 12:129-139.
- Zsóri, G., Illés, D., Terzin, V., Ivány, E., & Czakó, L. (2018). Exocrine pancreatic insufficiency in type 1 and type 2 diabetes mellitus: do we need to treat it? A systematic review. Pancreatology : official journal of the International Association of Pancreatology (IAP) … [et al.], 18(5), 559–565.
- Löhr JM, Dominguez-Munoz E, Rosendahl J, et al. United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU). United European Gastroenterol J. 2017;5(2):153-199.
- Víctor González-Sánchez, Rahma Amrani, Victoria González, Celia Trigo, Antonio Picó, Enrique de-Madaria, Diagnosis of exocrine pancreatic insufficiency in chronic pancreatitis: ¹³C-Mixed Triglyceride Breath Test versus Fecal Elastase, Pancreatology, Volume 17, Issue 4, 2017, Pages 580-585, ISSN 1424-3903.
- Gan, C., Chen, Y. H., Liu, L., Gao, J. H., Tong, H., Tang, C. W., & Liu, R. (2017). Efficacy and safety of pancreatic enzyme replacement therapy on exocrine pancreatic insufficiency: a meta-analysis. Oncotarget, 8(55), 94920–94931.
- Keller, J., Brückel, S., Jahr, C., & Layer, P. (2011). A modified ¹³C-mixed triglyceride breath test detects moderate pancreatic exocrine insufficiency. Pancreas, 40(8), 1201–1205.
- Domínguez-Muñoz, J. E., Iglesias-García, J., Vilariño-Insua, M., & Iglesias-Rey, M. (2007). ¹³C-mixed triglyceride breath test to assess oral enzyme substitution therapy in patients with chronic pancreatitis. Clinical gastroenterology and hepatology : the official clinical practice journal of the American Gastroenterological Association, 5(4), 484–488.
- Vantrappen, G R et al. “Mixed triglyceride breath test: a noninvasive test of pancreatic lipase activity in the duodenum.” Gastroenterology vol. 96,4 (1989): 1126-34.
Studies
Protocol Number: 2102CLI, Start: 2021
A 3-part Phase 1a/1b, first-in-human , randomized, double-blind, placebo-controlled study to evaluate safety, tolerability, and pharmacokinetics of single and multiple ascending doses of oral CDX 7108 in healthy adult subjects and to evaluate proof-of-concept via pharmacodynamics of a single dose of oral CDX 7108 in subjects with exocrine pancreatic insufficiency. Sponsor: Nestlé Suisse S.A.
Eudra CT Number: 2016-005063-13 Start: 2017, Duration/Value: completed
Investigation of Efficacy, Safety, Acid Resistance and Mode of Action of Lipases in Nortase and Kreon with the Pancreo-Lip ¹³C breath test in Subjects with Severe Exocrine Pancreatic Insufficiency. Sponsor: Repha GmbH
